(2-Methyl-2H-indazol-6-yl)boronic acid
CAS No.:
1001907-57-8
M. Wt:
175.98000
M. Fa:
C8H9BN2O2
InChI Key:
JADBVQWCMHPPQA-UHFFFAOYSA-N
Appearance:
Brown Solid
Names and Identifiers of (2-Methyl-2H-indazol-6-yl)boronic acid
CAS Number |
1001907-57-8 |
|---|---|
EC Number |
682-940-7 |
MDL Number |
MFCD09870057 |
IUPAC Name |
(2-methylindazol-6-yl)boronic acid |
InChI |
InChI=1S/C8H9BN2O2/c1-11-5-6-2-3-7(9(12)13)4-8(6)10-11/h2-5,12-13H,1H3 |
InChIKey |
JADBVQWCMHPPQA-UHFFFAOYSA-N |
Canonical SMILES |
B(C1=CC2=NN(C=C2C=C1)C)(O)O |
UNSPSC Code |
12352100 |
Physical and chemical properties of (2-Methyl-2H-indazol-6-yl)boronic acid
Boiling Point |
430.8ºC at 760 mmHg |
|---|---|
Density |
1.27 g/cm3 |
Exact Mass |
176.07600 |
Flash Point |
214.3ºC |
H Bond Acceptors |
3 |
H Bond Donors |
2 |
LogP |
1.315 |
Melting Point |
105-110°C |
Molecular Formula |
C8H9BN2O2 |
Molecular Weight |
175.98000 |
PSA |
58.28000 |
Storage condition |
2-8°C |
Safety Information of (2-Methyl-2H-indazol-6-yl)boronic acid
Applications of (2-Methyl-2H-indazol-6-yl)boronic acid
(2-Methyl-2H-indazol-6-yl)boronic acid finds applications in:
- Organic Synthesis: As a building block in synthesizing pharmaceuticals and agrochemicals.
- Material Science: In developing new materials through polymerization processes.
- Bioconjugation: In biochemistry for labeling and detecting biomolecules due to its reactive nature.
Its versatility in synthetic chemistry makes it a valuable reagent in both academic and industrial settings.
Interaction Studies of (2-Methyl-2H-indazol-6-yl)boronic acid
Studies on (2-Methyl-2H-indazol-6-yl)boronic acid interactions are primarily focused on its role in catalytic processes and enzyme inhibition. The compound's ability to form reversible covalent bonds suggests potential interactions with serine proteases and other enzymes that contain nucleophilic residues. Understanding these interactions is crucial for elucidating its biological mechanisms and enhancing its application in medicinal chemistry.
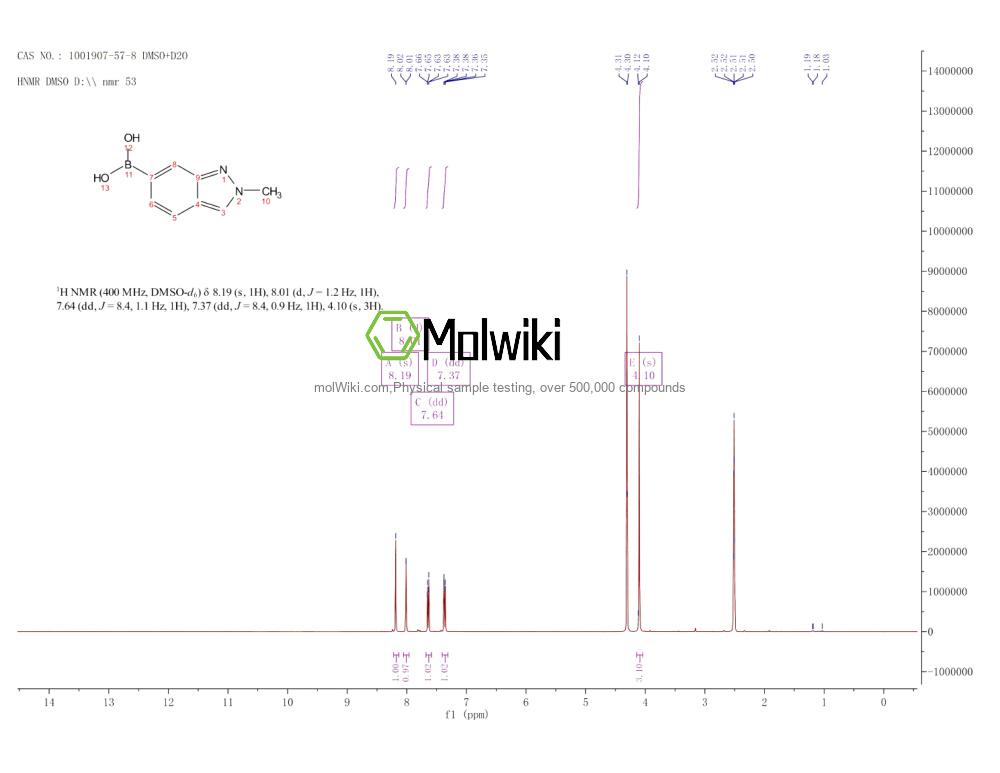
Physical sample testing spectrum (NMR) of (2-Methyl-2H-indazol-6-yl)boronic acid