tert-Butyl 2-amino-3-carbamoyl-4,5-dihydrothieno[2,3-c]pyridine-6(7H)-carboxylate
CAS No.:
1001020-08-1
M. Wt:
297.37300
M. Fa:
C13H19N3O3S
InChI Key:
RITBMKDFXNCZCM-UHFFFAOYSA-N
Appearance:
Pale-yellow Solid
Names and Identifiers of 1001020-08-1
CAS Number |
1001020-08-1 |
|---|---|
MDL Number |
MFCD11108853 |
IUPAC Name |
tert-butyl 2-amino-3-carbamoyl-5,7-dihydro-4H-thieno[2,3-c]pyridine-6-carboxylate |
InChI |
InChI=1S/C13H19N3O3S/c1-13(2,3)19-12(18)16-5-4-7-8(6-16)20-11(15)9(7)10(14)17/h4-6,15H2,1-3H3,(H2,14,17) |
InChIKey |
RITBMKDFXNCZCM-UHFFFAOYSA-N |
Canonical SMILES |
CC(C)(C)OC(=O)N1CCC2=C(C1)SC(=C2C(=O)N)N |
UNSPSC Code |
12352100 |
Physical and chemical properties of 1001020-08-1
Exact Mass |
297.11500 |
|---|---|
LogP |
2.94180 |
Molecular Formula |
C13H19N3O3S |
Molecular Weight |
297.37300 |
PSA |
126.89000 |
Storage condition |
2-8°C |
Safety Information of 1001020-08-1
Applications of 1001020-08-1
The applications of tert-butyl 2-amino-3-carbamoyl-4,5-dihydrothieno[2,3-c]pyridine-6(7H)-carboxylate span various fields:
- Pharmaceutical Development: As a lead compound in drug discovery targeting infectious diseases or cancer.
- Chemical Research: Serving as an intermediate in synthesizing more complex organic molecules.
- Biochemical Studies: Investigating the mechanisms of action of similar compounds in biological systems.
These applications highlight the compound's significance in both practical and research contexts .
Interaction Studies of 1001020-08-1
Interaction studies involving tert-butyl 2-amino-3-carbamoyl-4,5-dihydrothieno[2,3-c]pyridine-6(7H)-carboxylate focus on its binding affinity with various biological targets. These studies may include:
- Enzyme Inhibition Assays: Evaluating the compound's ability to inhibit specific enzymes related to disease pathways.
- Receptor Binding Studies: Assessing interactions with cellular receptors that mediate physiological responses.
Such studies are critical for understanding the pharmacodynamics and pharmacokinetics of the compound .
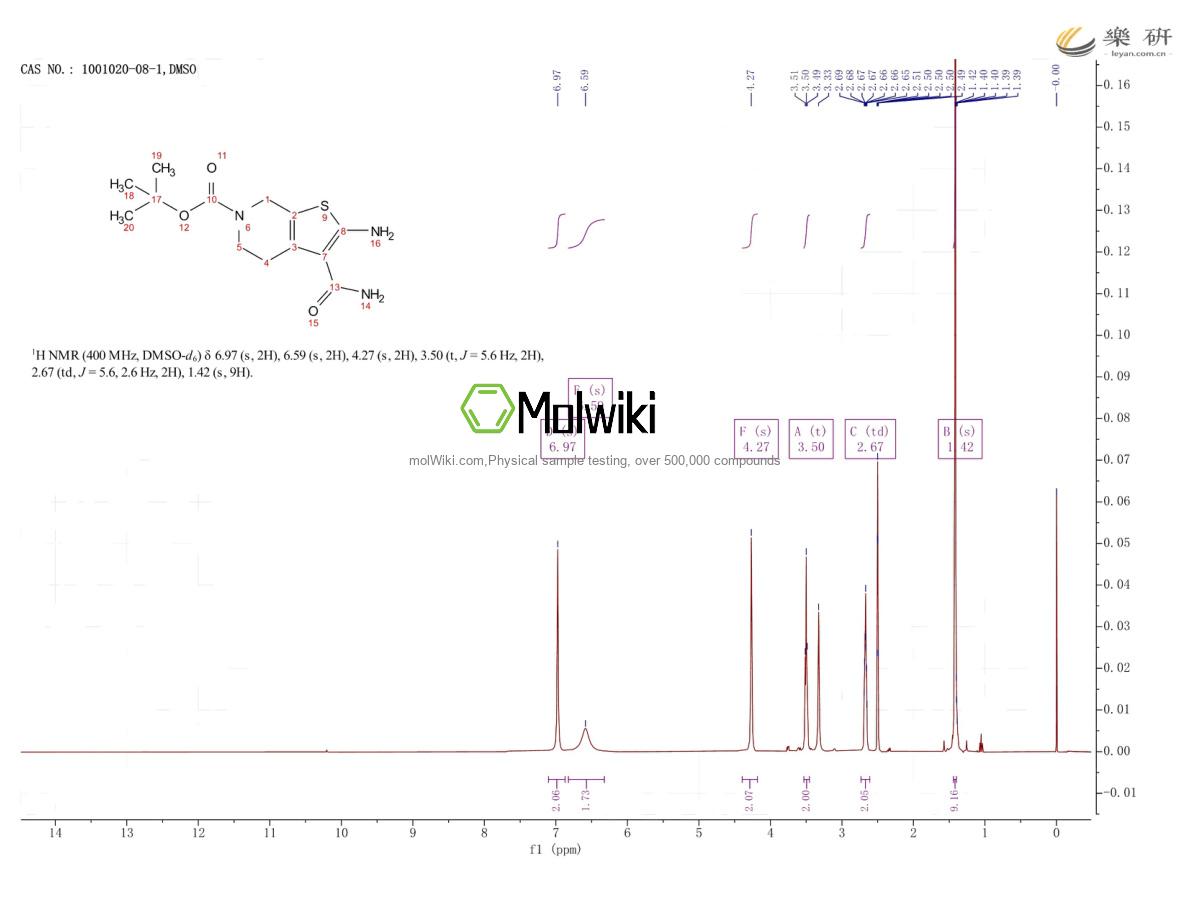
Physical sample testing spectrum (NMR) of 1001020-08-1