2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
Names and Identifiers of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
CAS Number |
1003711-04-3 |
|---|---|
MDL Number |
MFCD09835240 |
IUPAC Name |
2-amino-5-fluoropyridin-3-ol |
InChI |
InChI=1S/C5H5FN2O/c6-3-1-4(9)5(7)8-2-3/h1-2,9H,(H2,7,8) |
InChIKey |
QEDZENLHGZUFBK-UHFFFAOYSA-N |
Canonical SMILES |
NC1=NC=C(F)C=C1O |
UNSPSC Code |
12352100 |
Physical and chemical properties of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
Acidity coefficient |
11.49±0.10(Predicted) |
|---|---|
Boiling Point |
370.9±42.0 °C(Predicted) |
Density |
1.462±0.06 g/cm3(Predicted) |
Exact Mass |
128.03900 |
LogP |
1.08970 |
Molecular Formula |
C5H5FN2O |
Molecular Weight |
128.10400 |
PSA |
59.14000 |
Storage condition |
2-8°C |
Safety Information of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
Applications of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
2-Amino-5-fluoropyridin-3-ol finds applications in various fields:
- Pharmaceuticals: It serves as an intermediate in the synthesis of active pharmaceutical ingredients (APIs) targeting specific diseases.
- Agrochemicals: The compound is utilized in developing herbicides and pesticides due to its biological activity.
- Material Science: Its unique properties make it suitable for creating functional materials and coatings.
Interaction Studies of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
Studies on the interactions of 2-amino-5-fluoropyridin-3-ol with biomolecules have shown that it can bind effectively to certain enzymes, potentially inhibiting their activity. This binding affinity is attributed to the presence of the amino and hydroxyl groups, which can form hydrogen bonds with active sites on proteins or enzymes. Further research is needed to elucidate the specific pathways through which this compound exerts its biological effects.
Biological Activity of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE
Research indicates that 2-amino-5-fluoropyridin-3-ol exhibits various biological activities, including potential antimicrobial and antifungal properties. The presence of both amino and hydroxyl groups enhances its ability to interact with biological targets, making it a candidate for further investigation in drug development. Its structural features may contribute to its binding affinity to specific enzymes or receptors, which is crucial for its biological effects.
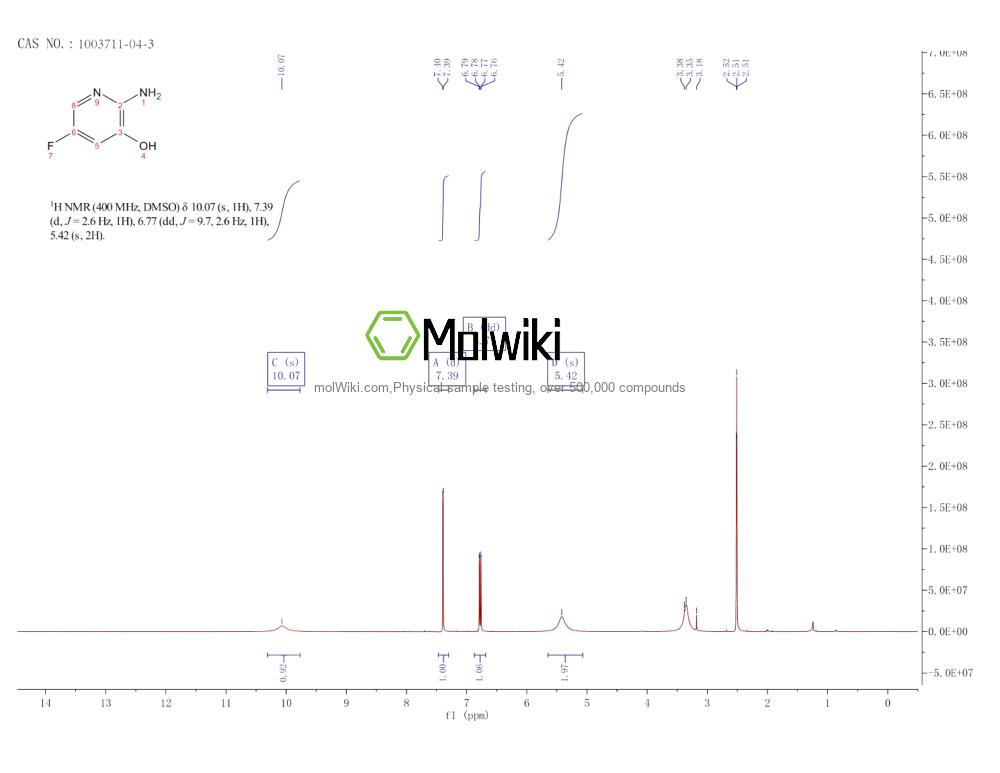
Physical sample testing spectrum (NMR) of 2-AMINO-5-FLUORO-3-HYDROXYPYRIDINE