2-Fluoro-3-hydroxybenzonitrile
CAS No.:
1000339-24-1
M. Wt:
137.11100
M. Fa:
C7H4FNO
InChI Key:
CVJVSXKZGFQWJK-UHFFFAOYSA-N
Appearance:
Yellow Solid
Names and Identifiers of 2-Fluoro-3-hydroxybenzonitrile
CAS Number |
1000339-24-1 |
|---|---|
MDL Number |
MFCD09839222 |
IUPAC Name |
2-fluoro-3-hydroxybenzonitrile |
InChI |
InChI=1S/C7H4FNO/c8-7-5(4-9)2-1-3-6(7)10/h1-3,10H |
InChIKey |
CVJVSXKZGFQWJK-UHFFFAOYSA-N |
Canonical SMILES |
C1=CC(=C(C(=C1)O)F)C#N |
UNSPSC Code |
12352100 |
Physical and chemical properties of 2-Fluoro-3-hydroxybenzonitrile
Acidity coefficient |
7.32±0.10(Predicted) |
|---|---|
Boiling Point |
232.0±25.0 °C(Predicted) |
Density |
1.34±0.1 g/cm3(Predicted) |
Exact Mass |
137.02800 |
LogP |
1.40298 |
Molecular Formula |
C7H4FNO |
Molecular Weight |
137.11100 |
PSA |
44.02000 |
Storage condition |
Sealed in dry,Room Temperature |
Safety Information of 2-Fluoro-3-hydroxybenzonitrile
Applications of 2-Fluoro-3-hydroxybenzonitrile
2-Fluoro-3-hydroxybenzonitrile finds applications across various domains:
- Pharmaceuticals: It serves as an intermediate in the synthesis of biologically active molecules.
- Materials Science: The compound may be utilized in developing new materials with specific electronic or optical properties due to its unique structure.
- Research Tools: It is also used in proteomics research as a biochemical agent.
Interaction Studies of 2-Fluoro-3-hydroxybenzonitrile
Interaction studies are crucial for understanding how 2-Fluoro-3-hydroxybenzonitrile behaves in biological systems:
- Protein Binding Studies: These studies assess how well the compound interacts with proteins, which is essential for predicting its pharmacokinetic properties.
- Receptor Interaction: Investigating how this compound interacts with specific receptors can provide insights into its potential therapeutic uses.
Such studies are vital for advancing the understanding of this compound's utility in drug development.
Biological Activity of 2-Fluoro-3-hydroxybenzonitrile
Research indicates that compounds containing fluorine often exhibit enhanced biological activity compared to their non-fluorinated counterparts. 2-Fluoro-3-hydroxybenzonitrile has been studied for its potential roles in medicinal chemistry. Specifically:
- Antimicrobial Activity: Some studies suggest that fluorinated compounds can possess antimicrobial properties, making them candidates for developing new antibiotics.
- Pharmacological Potential: The unique electronic properties imparted by the fluorine atom may enhance the binding affinity of this compound to biological targets.
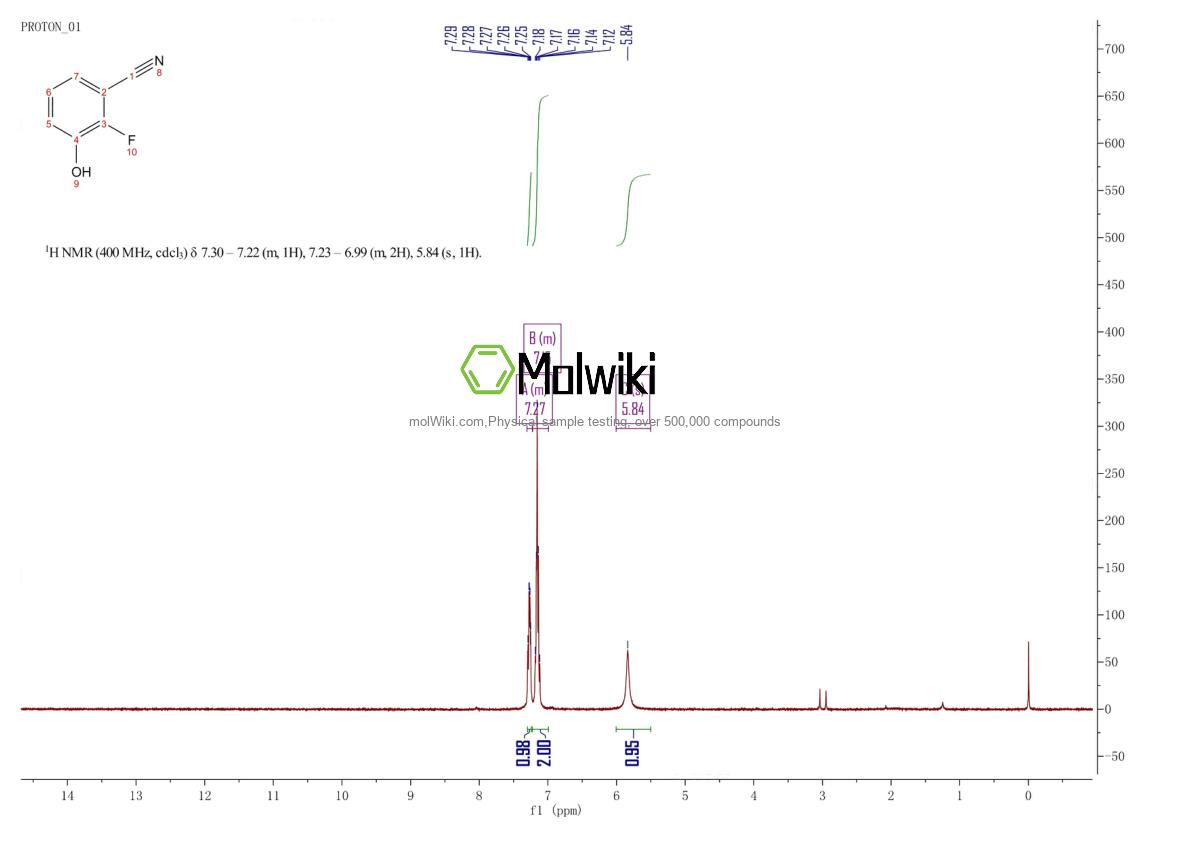
Physical sample testing spectrum (NMR) of 2-Fluoro-3-hydroxybenzonitrile