6-Bromo-4-fluoroindolin-2-one
Names and Identifiers of 6-Bromo-4-fluoroindolin-2-one
CAS Number |
1000341-00-3 |
|---|---|
MDL Number |
MFCD09880178 |
IUPAC Name |
6-bromo-4-fluoro-1,3-dihydroindol-2-one |
InChI |
InChI=1S/C8H5BrFNO/c9-4-1-6(10)5-3-8(12)11-7(5)2-4/h1-2H,3H2,(H,11,12) |
InChIKey |
UNVVSMMNEZBZDQ-UHFFFAOYSA-N |
Canonical SMILES |
C1C2=C(C=C(C=C2F)Br)NC1=O |
UNSPSC Code |
12352100 |
Physical and chemical properties of 6-Bromo-4-fluoroindolin-2-one
Boiling Point |
261.6±50.0 °C at 760 mmHg |
|---|---|
Density |
1.9±0.1 g/cm3 |
Exact Mass |
226.938202 |
Flash Point |
112.0±30.1 °C |
H Bond Acceptors |
1 |
H Bond Donors |
1 |
Index of Refraction |
1.673 |
LogP |
0.35 |
Molecular Formula |
C8H3BrFNO |
Molecular Weight |
228.018 |
PSA |
29.43000 |
Vapour Pressure |
0.0±0.5 mmHg at 25°C |
Safety Information of 6-Bromo-4-fluoroindolin-2-one
Applications of 6-Bromo-4-fluoroindolin-2-one
6-Bromo-4-fluoroindolin-2-one has several applications:
- Medicinal Chemistry: It serves as a building block for synthesizing pharmaceuticals targeting various diseases, particularly cancers.
- Organic Synthesis: The compound is used as a reagent in organic synthesis due to its ability to undergo diverse chemical transformations.
- Research: It is utilized in biological studies to explore its potential therapeutic effects and mechanisms of action.
Interaction Studies of 6-Bromo-4-fluoroindolin-2-one
Studies on the interactions of 6-Bromo-4-fluoroindolin-2-one have shown that it can inhibit certain enzymes involved in drug metabolism, such as cytochrome P450 isoforms. Specifically, it acts as an inhibitor for CYP1A2 while showing no significant inhibition on other isoforms like CYP2C19 or CYP3A4. This selectivity may enhance its therapeutic profile by reducing potential drug-drug interactions.
Biological Activity of 6-Bromo-4-fluoroindolin-2-one
6-Bromo-4-fluoroindolin-2-one exhibits notable biological activities. Research indicates that derivatives of this compound may possess antitumor and antimicrobial properties. Its mechanism of action often involves interaction with molecular targets such as receptor tyrosine kinases, leading to inhibition of signaling pathways associated with cancer cell proliferation and angiogenesis.
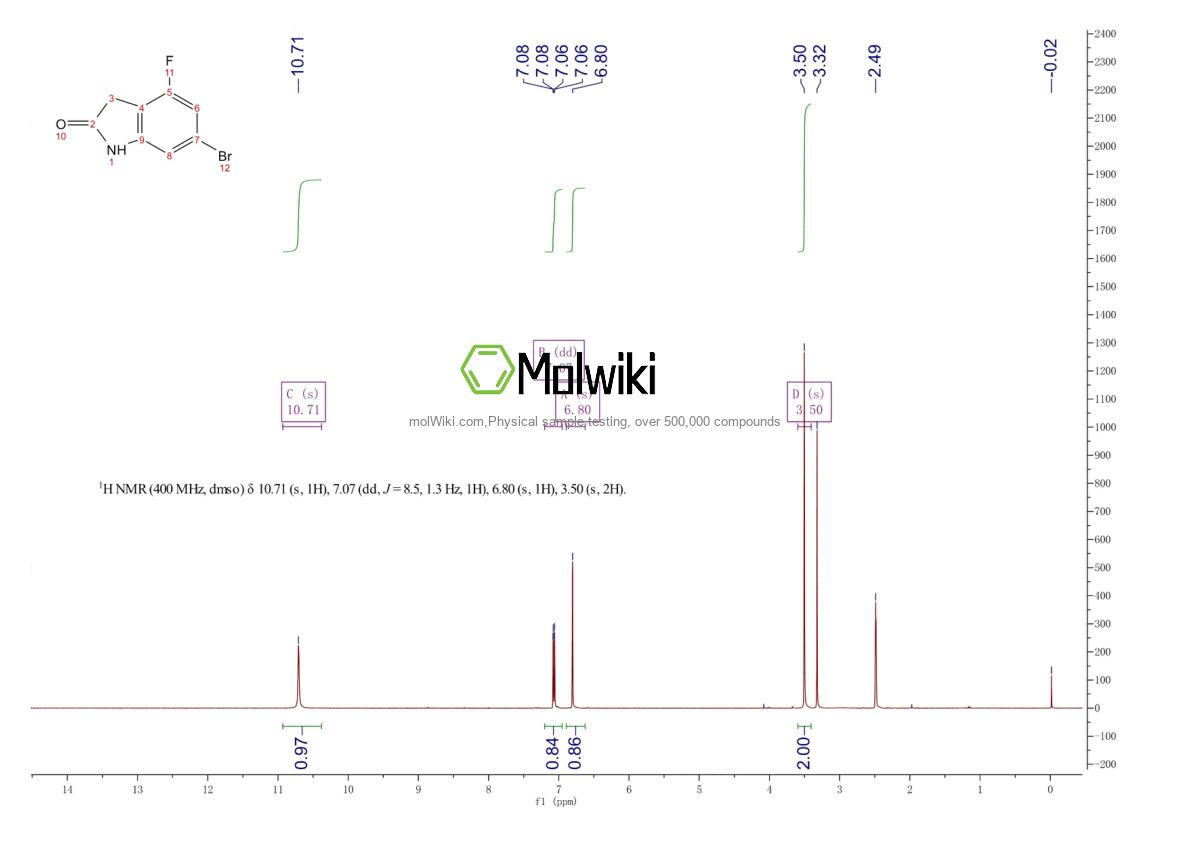
Physical sample testing spectrum (NMR) of 6-Bromo-4-fluoroindolin-2-one