Methyl 3-amino-5-bromo-2-methylbenzoate
CAS No.:
1000342-11-9
M. Wt:
244.085
M. Fa:
C9H10BrNO2
InChI Key:
NMLOSXSDLWFBKT-UHFFFAOYSA-N
Appearance:
Pale-yellow Solid
Names and Identifiers of Methyl 3-amino-5-bromo-2-methylbenzoate
CAS Number |
1000342-11-9 |
|---|---|
EC Number |
811-830-5 |
MDL Number |
MFCD08690071 |
IUPAC Name |
methyl 3-amino-5-bromo-2-methylbenzoate |
InChI |
InChI=1S/C9H10BrNO2/c1-5-7(9(12)13-2)3-6(10)4-8(5)11/h3-4H,11H2,1-2H3 |
InChIKey |
NMLOSXSDLWFBKT-UHFFFAOYSA-N |
Canonical SMILES |
CC1=C(C=C(C=C1N)Br)C(=O)OC |
UNSPSC Code |
12352100 |
Physical and chemical properties of Methyl 3-amino-5-bromo-2-methylbenzoate
Acidity coefficient |
2.23±0.10(Predicted) |
|---|---|
Boiling Point |
336.0±37.0 °C at 760 mmHg |
Density |
1.5±0.1 g/cm3 |
Exact Mass |
242.989487 |
Flash Point |
157.0±26.5 °C |
Index of Refraction |
1.591 |
LogP |
2.55 |
Melting Point |
52 °C |
Molecular Formula |
C9H10BrNO2 |
Molecular Weight |
244.085 |
PSA |
52.32000 |
Solubility |
soluble in Methanol |
Storage condition |
Keep in dark place,Sealed in dry,Room Temperature |
Vapour Pressure |
0.0±0.7 mmHg at 25°C |
Safety Information of Methyl 3-amino-5-bromo-2-methylbenzoate
Applications of Methyl 3-amino-5-bromo-2-methylbenzoate
Methyl 3-amino-5-bromo-2-methylbenzoate has several applications:
- Pharmaceuticals: It may serve as an intermediate in the synthesis of bioactive molecules.
- Research: Used in studies exploring structure-activity relationships in medicinal chemistry.
- Chemical Synthesis: Acts as a building block for more complex organic compounds.
Interaction Studies of Methyl 3-amino-5-bromo-2-methylbenzoate
Interaction studies involving methyl 3-amino-5-bromo-2-methylbenzoate focus on its reactivity with other chemical agents and biological systems. These studies are crucial for understanding its potential therapeutic roles and safety profiles. For instance, examining its interactions with enzymes or receptors can provide insights into its pharmacological properties.
Biological Activity of Methyl 3-amino-5-bromo-2-methylbenzoate
Research on methyl 3-amino-5-bromo-2-methylbenzoate indicates potential biological activities, particularly in medicinal chemistry. Compounds with similar structures often exhibit:
- Antimicrobial Properties: Many derivatives show activity against various bacteria and fungi.
- Anti-inflammatory Effects: Some studies suggest that similar compounds may help alleviate inflammation.
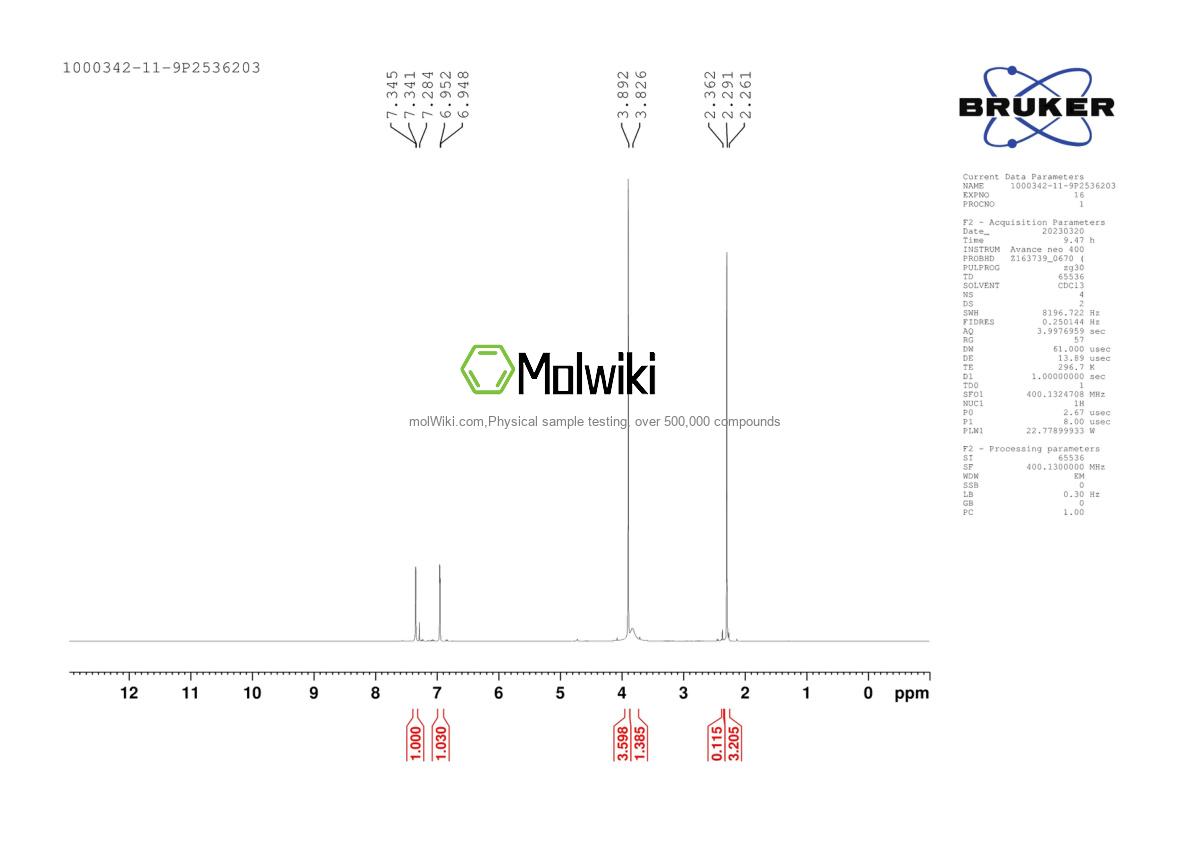
Physical sample testing spectrum (NMR) of Methyl 3-amino-5-bromo-2-methylbenzoate